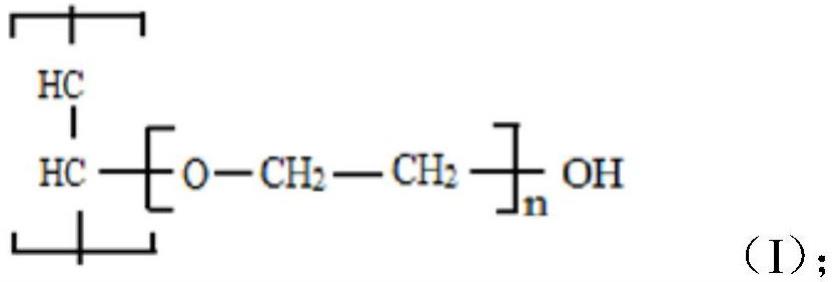Polyvinyl alcohol (PVA) is one of the most important and widely used water-soluble polymers in industrial applications. Its preparation process involves first polymerizing vinyl acetate (VAc) to form polyvinyl acetate (PVAc). The vinyl acetate groups (-OAc) on the PVAc are then converted to hydroxyl groups (-OH) through an alcoholysis (hydrolysis) reaction. Based on the degree of alcoholysis, PVA is divided into two major series: fully hydrolyzed and partially hydrolyzed.

Fully hydrolyzed 99 series PVA (such as ElephChem pva 2699, 2499, 2099, and 1799) refers to grades with a degree of hydrolysis of 99.0 mol% or higher. This extremely high degree of hydrolysis is the core prerequisite for these PVA grades to achieve high performance, strength, and water resistance. This blog will analyze, from four perspectives: molecular structure, grade differentiation, performance advantages, and key application areas, how fully hydrolyzed 99 Series PVA has become the cornerstone of "hardcore" materials such as high-performance fibers, specialty films, and durable adhesives.
1.Molecular Structure Determine Performance: The Mechanism and Effect of Complete Hydrolysis
1.1 Hydroxyl Density and Hydrogen Bonding Network Construction
In the fully hydrolyzed 99 Series, nearly all hydrophobic vinyl acetate groups on the molecular chain are replaced by hydrophilic hydroxyl groups. Hydroxyl groups (-OH) are extremely polar functional groups that form strong intramolecular and intermolecular hydrogen bonds, building a highly dense and stable three-dimensional network.
This dense hydrogen-bonding network contributes to two crucial molecular effects:
- High crystallinity: Strong hydrogen bonding forces enable PVA molecular chains to stack neatly and tightly, forming highly ordered crystalline regions. This increased crystallinity is the fundamental reason for the high tensile strength and high modulus of 99 Series PVA.
- Water Resistance: The dense hydrogen bond network makes it difficult for external water molecules to penetrate the crystals at room temperature and disrupt the connections between the molecular chains, effectively preventing PVA from dissolving. Therefore, 99 series PVA is essentially insoluble in water at room temperature and typically requires hot water above 90°C to fully dissolve and disperse. This ensures its structural stability in humid environments and aqueous systems.
1.2 Linear Correlation between Degree of Polymerization and Viscosity/Strength
Assuming a constant degree of hydrolysis (HD>99.0%), the differences between fully hydrolyzed 99 series PVA grades are primarily determined by the average degree of polymerization (DP) or average molecular weight (MW). DP is a key parameter that determines the rheological properties of polymer solutions and the mechanical properties of the final product.
The DP ladder of ElephChem 99 series grades (based on the average DP):
- Ultra-High DP (Polyvinyl Alcohol 2699): DP = 2600-3000. These grades have the longest molecular chains and the highest degree of chain entanglement. Its highest solution viscosity imparts exceptional cohesive strength and adhesion to the cured material, making it an ideal choice for manufacturing high-strength, high-modulus fibers and specialty high-viscosity adhesives.
- Medium-high degree of polymerization (Polyvinyl Alcohol 2499 / Polyvinyl Alcohol 2099): DP = 2,000-2,500. This grade offers a balanced viscosity and mechanical properties. It is the most widely used grade for sizing agents in the textile industry and for general-purpose, high-performance coatings and films.
- Medium-low degree of polymerization (Polyvinyl Alcohol 1799): DP = 1,700-1,800. Its relatively low solution viscosity facilitates its use in systems with high solids content or requiring rapid penetration. For example, precursors for polyvinyl butyral (PVB) require precise molecular weight control (e.g., 1799 for PVB, MW = 76,000-82,000) to ensure efficient acetalization and the quality of the resulting interlayer film.
2. Core Performance Advantages of the Fully Hydrolyzed PVA 99 Series
- Excellent Mechanical Properties (High Strength, High Modulus): High crystallinity gives PVA high tensile strength and modulus. Wet or dry-wet spinning yields high-strength, high-modulus PVA fibers with properties comparable to those of ultra-high-density polyethylene (UHMWPE). These fibers are a key raw material for replacing asbestos in cement reinforcement and ballistic materials.
- Excellent Gas Barrier Properties: PVA films, particularly those produced from the 99 series, offer one of the best barrier properties against gases like oxygen and nitrogen among known polymer materials. The highly hydrogen-bonded network within their molecular structure prevents gas permeation, making them ideal as high-performance barrier layers for oxygen-sensitive food and pharmaceutical packaging.
- Chemical and Oil Resistance: The 99 series PVA shows good resistance to solvents, oils, greases, and weak acids and bases because its molecules are very stable and it has few non-crystalline areas. This makes it useful for industrial coatings and special glues.
- Thermal stability: High crystallinity gives 99 series PVA a higher glass transition temperature (Tg) and melting temperature (Tm), improving the material's resistance to heat deformation and upper temperature limit.
3. Analysis of Key Industrial Applications of Fully Hydrolyzed 99 Series PVA
The unique properties of 99 Series PVA make it irreplaceable in multiple high-value-added sectors:
3.1 High-Strength High-Modulus PVA Fiber (HTHM PVA Fiber)
This is one of the most valuable end-products of 99 Series PVA. For example, the 1799 grade, with a DP of approximately 1750, achieves a high degree of molecular orientation through specialized spinning, heat treatment, and stretching processes.
- Applications: Used to replace asbestos and steel mesh in construction, it reinforces cement, mortar, and concrete, significantly improving the material's impact resistance, freeze-thaw resistance, and fatigue resistance. It is widely used in civil engineering structures such as highways, water conservancy projects, tunnel linings, and cement slabs.
3.2 Textile and Paper Industry
- Textile Warp Sizing: High-polymerization grades such as 2499 and 2699 provide an extremely tough and smooth size film, significantly improving the abrasion resistance and breaking strength of warp yarns during weaving. They are the preferred size for high-density, high-count fabrics (such as denim and premium cotton).
- Papermaking Surface Sizing Agent: As a surface sizing agent, the 99 series PVA forms a high-strength film on the surface of paper, significantly improving its surface strength, folding resistance, and printability. This is crucial for high-end coated paper and specialty functional papers (such as thermal paper and dust-free paper).
3.3 Polyvinyl Butyral (PVB) Precursor
PVB is a core material for automotive safety glass and architectural laminated glass. As an intermediate in the acetalization reaction, the quality of PVA directly determines the optical clarity, toughness, adhesion, and aging resistance of the final PVB film. Grades: 1799 specialty grades (such as SX-I/II/III) with a DP ≈ 1700-1850 are precisely designed to ensure ideal molecular structure and uniform dispersion during the subsequent acetalization reaction, meeting the stringent optical quality requirements of safety glass.
3.4 High-Performance Building Adhesives and Dry-Mix Mortars
In the construction industry, 99-series PVAs are used as high-performance additives to improve material durability and adhesion.
- Applications: As secondary dispersing binders and water-retaining agents in mortars and putty powders, their high bond strength and water resistance ensure the stability and durability of wall putties, tile adhesives, and other materials in humid and temperature-stable environments.
4. Conclusion: Future Outlook for Fully Hydrolyzed 99-Series PVA
99-series PVAs are a classic and promising branch of polymer materials science. By precisely controlling the degree of hydrolysis and polymerization, as demonstrated by ElephChem's grade system, the industry can develop specialized grades tailored to meet the demands of diverse and demanding applications.
From high-strength fibers that reinforce modern infrastructure, to PVB interlayer films that ensure safety, to environmentally friendly, high-performance coatings that enhance quality of life, the 99 series PVA, with its unparalleled strength, stability, and water resistance, continues to play a key role as a driver of high-performance, "hardcore" materials in the upgrading and sustainable development of the global manufacturing industry. As novel uses, like 3D printing and medical hydrogels, ask for better PVA, studies into improving and changing the 99 series PVA will likely increase. This will probably expand its value in industry and its market potential.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com